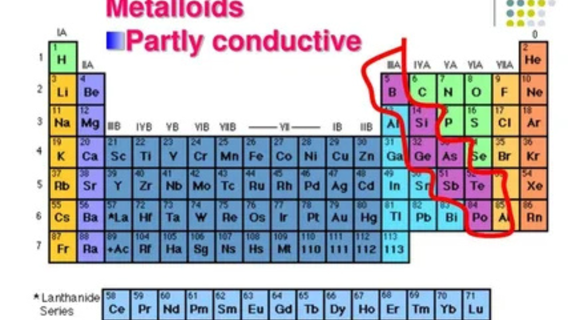
Metalloids, also known as semimetals, are elements that exhibit properties between metals and nonmetals. Their unique characteristics make them incredibly valuable in a wide range of industries and applications. Located along the zig-zag line of the periodic table, metalloids share traits of both metals and nonmetals, such as electrical conductivity, hardness, and the ability to form alloys. In this article, we'll explore the common uses of metalloids in various sectors, from electronics to medicine.
What Are Metalloids?
Metalloids are elements that typically have a shiny appearance like metals, but are brittle and often poor conductors of heat and electricity, resembling nonmetals in some respects. Common metalloids include silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), and tellurium (Te). Their diverse properties make them essential in many applications, particularly in the field of electronics and technology.
1. Semiconductors in Electronics
Perhaps the most well-known and significant use of metalloids is in the manufacturing of semiconductors. Silicon is the star element in this domain, used extensively in the production of microchips, transistors, and solar cells. As a semiconductor, silicon can conduct electricity under certain conditions, making it ideal for controlling electrical current in devices such as computers, smartphones, and televisions.
Germanium, another metalloid, is also used in semiconductors. It was once widely used in early transistors and is still used in some modern electronics, such as infrared optics and fiber-optic communication systems, due to its ability to transmit signals effectively.
2. Solar Cells and Renewable Energy
Metalloids play a crucial role in the development of renewable energy technologies, particularly solar power. Silicon is the primary material used in photovoltaic (PV) cells, which convert sunlight into electricity. Solar panels, found on rooftops and solar farms, rely on silicon's ability to absorb and convert sunlight efficiently. As the demand for clean, renewable energy increases, silicon-based solar technology remains a key player in reducing dependence on fossil fuels.
3. Alloys and Metal Alloys
Certain metalloids are used to improve the properties of alloys. For example, silicon is added to steel to enhance its strength and durability. This is particularly important in the construction of buildings, bridges, and vehicles, where high-strength materials are required to withstand heavy loads and environmental stress.
Antimony is another metalloid used in alloys, particularly in lead-based alloys for batteries. Antimony improves the durability and performance of lead-acid batteries, which are commonly used in cars and other vehicles.
4. Glass Production and Optics
Metalloids such as boron and silicon are integral to the production of high-quality glass. Borosilicate glass, which is known for its durability and resistance to thermal shock, is commonly used in laboratory equipment, cookware, and high-end optics. Silicon dioxide, or silica, is a key ingredient in making regular glass for windows, bottles, and other everyday products.
In the field of optics, germanium is utilized in infrared lenses and cameras. Its transparency to infrared radiation makes it ideal for applications in night-vision systems, infrared cameras, and thermal imaging devices.
5. Pharmaceutical and Medical Applications
Metalloids also have a place in medicine, particularly in the form of drugs and treatments. Arsenic, despite its toxic reputation, has been used in small doses for medical purposes, such as the treatment of certain cancers, including leukemia. Arsenic trioxide, a compound derived from arsenic, is used in chemotherapy treatments for cancer patients.
Tellurium is another metalloid with potential medical uses. It has been investigated for its antibacterial and antifungal properties, and some research suggests that tellurium compounds may play a role in treating infections and improving immune system responses.
6. Fire Retardants and Pesticides
Certain metalloids are also used in fire retardants and pesticides. Antimony compounds are commonly added to materials such as textiles, plastics, and foams to make them more resistant to flames. This application is particularly important in the manufacture of furniture, curtains, and safety equipment, where fire resistance is critical.
Arsenic, though toxic, has been used historically in pesticides and wood preservatives. While its use in agriculture has decreased due to safety concerns, it remains a key component in some industrial applications.
7. Alloys in Batteries
Metalloids such as antimony and tellurium are also found in batteries. In particular, antimony is used in lead-acid batteries, improving their charge capacity and lifespan. These batteries are widely used in vehicles, backup power systems, and emergency lighting.
Conclusion
Metalloids common uses for metalloids of elements with unique properties that make them indispensable in many industries. From semiconductors and solar cells to alloys and medical applications, their versatility plays a crucial role in shaping modern technology and improving our daily lives. As research continues to uncover new uses for these elements, we can expect metalloids to remain at the forefront of innovation in fields ranging from electronics to renewable energy.
Whether you're working with electronics, energy production, or healthcare, understanding the importance of metalloids is essential to recognizing their value in a rapidly advancing technological world.
0 comments
Be the first to comment!
This post is waiting for your feedback.
Share your thoughts and join the conversation.
