Mastering the Lewis structure of molecules is essential for understanding their chemical properties and behaviors. Carbon tetrachloride, or CCl4, is a common compound encountered in chemistry education and various industrial applications. Knowing how to quickly solve its Lewis structure can be immensely beneficial for students and professionals alike. In this article, we'll explore step-by-step instructions and tips to efficiently solve the Lewis structure of CCl4, enabling you to grasp its molecular arrangement with ease.
Understanding the Basics of Lewis Structures
Before delving into the specifics of solving the Lewis structure of CCl4, let's briefly review the fundamentals of Lewis structures:
Valence Electrons: Valence electrons are the outermost electrons of an atom, responsible for bonding and chemical reactions.
Octet Rule: Most atoms tend to form bonds in such a way that they achieve a full outer shell of eight electrons, known as the octet rule.
Shared Electrons: Atoms can share electrons to achieve a stable electron configuration, forming covalent bonds.
Step-by-Step Guide to Solving the Lewis Structure of CCl4
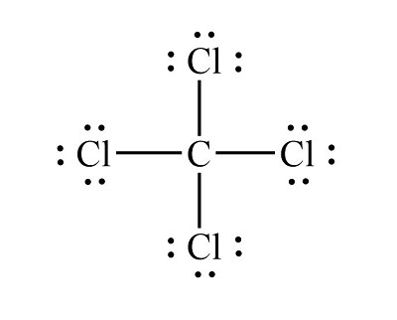
Count Valence Electrons: Carbon (C) has four valence electrons, while each chlorine (Cl) atom has seven valence electrons. The total number of valence electrons in CCl4 is therefore 4 (from C) + 4 × 7 (from Cl) = 32.
Arrange Atoms: Place the carbon atom in the center and surround it with four chlorine atoms, ensuring that each chlorine atom forms a single bond with the carbon atom.
Distribute Electrons: Begin by placing two electrons between the carbon atom and each chlorine atom to form single bonds. This accounts for 8 electrons (2 for each Cl-C bond), leaving 24 electrons remaining.
Complete Octets: Distribute the remaining electrons around the chlorine atoms to complete their octets. Each chlorine atom requires six more electrons to achieve a full octet, resulting in three lone pairs of electrons around each chlorine atom.
Verify Formal Charges: Calculate the formal charges of each atom to ensure the stability of the Lewis structure. The formal charge of an atom is calculated using the formula: Formal Charge = (Number of Valence Electrons) - (Number of Lone Pair Electrons) - (Number of Bonding Electrons).
Tips and Tricks for Quick Solving
Identify Central Atom: In most molecules, the least electronegative atom is placed in the center. In the case of CCl4, carbon is the central atom as it is less electronegative than chlorine.
Prioritize Single Bonds: Start by forming single bonds between the central atom and surrounding atoms before distributing lone pairs.
Check Octet Rule: Ensure that each atom in the Lewis structure, excluding hydrogen, achieves a full octet of electrons.
Solving the Lewis structure of CCl4 quickly and accurately is essential for understanding its molecular geometry and chemical behavior. By following the step-by-step guide and employing tips and tricks outlined in this article, you can efficiently determine the arrangement of atoms and electrons in carbon tetrachloride. Remember to practice solving Lewis structures of other molecules to enhance your proficiency in this fundamental aspect of chemistry. With a solid understanding of Lewis structures, you'll be well-equipped to tackle more complex chemical concepts and applications with confidence.
0 comments
Be the first to comment!
This post is waiting for your feedback.
Share your thoughts and join the conversation.
